Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Dalton Transactions (Internet), 50(33), p.11390 - 11397, 2021/09
Times Cited Count:2 Percentile:20.72(Chemistry, Inorganic & Nuclear)no abstracts in English
 O
O -type Schiff base complexes of tetravalent f-elements (M(IV) = Ce, Th, U, Np, and Pu) in solid state and in solution
-type Schiff base complexes of tetravalent f-elements (M(IV) = Ce, Th, U, Np, and Pu) in solid state and in solutionRadoske, T.*; Kloditz, R.*; Fichter, S.*; M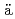 rz, J.*; Kaden, P.*; Patzschke, M.*; Schmidt, M.*; Stumpf, T.*; Walter, O.*; Ikeda, Atsushi
rz, J.*; Kaden, P.*; Patzschke, M.*; Schmidt, M.*; Stumpf, T.*; Walter, O.*; Ikeda, Atsushi
Dalton Transactions (Internet), 49(48), p.17559 - 17570, 2020/12
Times Cited Count:11 Percentile:64.17(Chemistry, Inorganic & Nuclear) -radiation and H
-radiation and H O
O induced oxidative dissolution of uranium(IV) oxide in aqueous solution containing phthalic acid
induced oxidative dissolution of uranium(IV) oxide in aqueous solution containing phthalic acidKumagai, Yuta; Jonsson, M.*
Dalton Transactions (Internet), 49(6), p.1907 - 1914, 2020/02
Times Cited Count:0 Percentile:0.01(Chemistry, Inorganic & Nuclear)This study aims to reveal possible involvements of organic acids in the oxidative dissolution of UO . Using phthalic acid as a model compound, we have measured adsorption on UO
. Using phthalic acid as a model compound, we have measured adsorption on UO and investigated effects on the reaction between H
and investigated effects on the reaction between H O
O and UO
and UO and on oxidative dissolution induced by
and on oxidative dissolution induced by  -irradiation. Significant adsorption of phthalic acid was observed even at neutral pH. However, the reaction between H
-irradiation. Significant adsorption of phthalic acid was observed even at neutral pH. However, the reaction between H O
O and UO
and UO in phthalic acid solution induced oxidative dissolution of U(VI) similarly to aqueous bicarbonate solution. These results indicate that even though phthalic acid adsorbs on the UO
in phthalic acid solution induced oxidative dissolution of U(VI) similarly to aqueous bicarbonate solution. These results indicate that even though phthalic acid adsorbs on the UO surface, it is not involved in the interfacial reaction by H
surface, it is not involved in the interfacial reaction by H O
O . In contrast, the dissolution of U by irradiation was inhibited in aqueous phthalic acid solution, whereas H
. In contrast, the dissolution of U by irradiation was inhibited in aqueous phthalic acid solution, whereas H O
O generated by radiolysis was consumed by UO
generated by radiolysis was consumed by UO . The inhibition suggests that radical species derived from phthalic acid was involved in the redox reaction process of UO
. The inhibition suggests that radical species derived from phthalic acid was involved in the redox reaction process of UO .
.
Nakane, Tomohiro*; Yoneyama, Shota*; Kodama, Takeshi*; Kikuchi, Koichi*; Nakao, Akiko*; Ohara, Takashi; Higashinaka, Ryuji*; Matsuda, Tatsuma*; Aoki, Yuji*; Fujita, Wataru*
Dalton Transactions (Internet), 48(1), p.333 - 338, 2019/01
Times Cited Count:2 Percentile:11.08(Chemistry, Inorganic & Nuclear)Kimura, Taiki*; Kaneko, Masashi; Watanabe, Masayuki; Miyashita, Sunao*; Nakashima, Satoru*
Dalton Transactions (Internet), 47(42), p.14924 - 14931, 2018/11
Times Cited Count:9 Percentile:48.79(Chemistry, Inorganic & Nuclear)We demonstrated density functional calculations of Eu(III) and Am(III) complexes with pnictogen-donor (X) ligands, CH )
) X-CH
X-CH -CH
-CH -X(CH
-X(CH )
) (X = N, P, As and Sb). We investigated the optimized structures of the cmoplexes and the Gibbs energy differences in the complex formation reactions. Those results indicated that the N- and P-donor ligands have Am(III) ion selectivity over Eu(III) ion, especially, the P-donor ligand showed the highest selectivity. The tendency of the Am(III)/Eu(III) selectivity by the pnictogen-dono ligands was found to be comparable to that of soft acid classification in hard and soft acids and bases rule. Mulliken's spin population analysis indicated that the bonding property between the metal ion and the pnictogen atoms correlated with the Am(III)/Eu(III) selectivity. In particular, the participation of f-orbital electrons of the metal ion in the covalency was indicated to have an important role for the selectivity.
(X = N, P, As and Sb). We investigated the optimized structures of the cmoplexes and the Gibbs energy differences in the complex formation reactions. Those results indicated that the N- and P-donor ligands have Am(III) ion selectivity over Eu(III) ion, especially, the P-donor ligand showed the highest selectivity. The tendency of the Am(III)/Eu(III) selectivity by the pnictogen-dono ligands was found to be comparable to that of soft acid classification in hard and soft acids and bases rule. Mulliken's spin population analysis indicated that the bonding property between the metal ion and the pnictogen atoms correlated with the Am(III)/Eu(III) selectivity. In particular, the participation of f-orbital electrons of the metal ion in the covalency was indicated to have an important role for the selectivity.